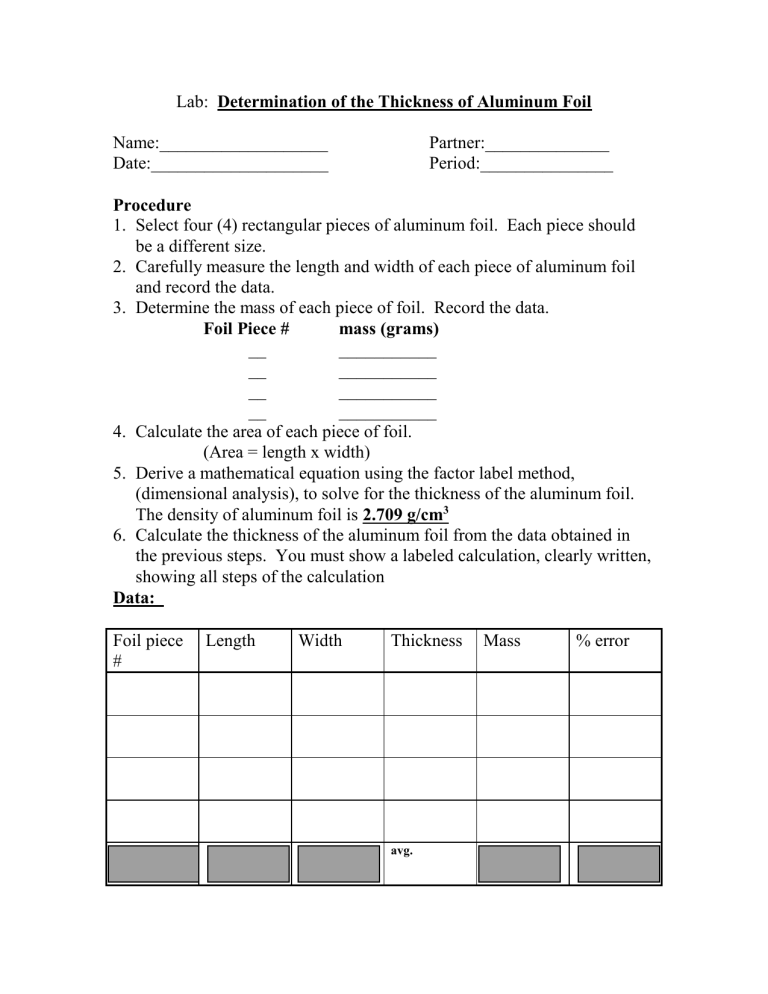
b) The density of aluminum is 2.70 g/cm3. The thickness of a rectangular sheet of aluminum foil - Brainly.com
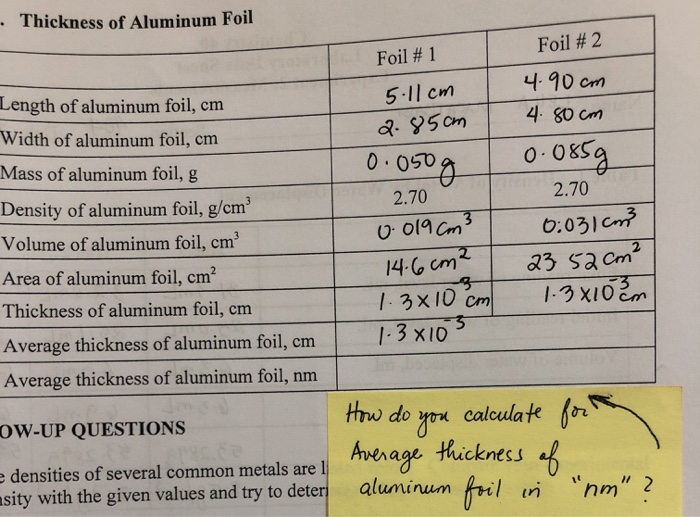
![SOLVED: The Thickness of Aluminum Foil Mass of Al Foil: 0.146 g Length of Al Foil: 5.99 cm Width of Al Foil: [missing value] Data Analysis Use your measured mass and volume SOLVED: The Thickness of Aluminum Foil Mass of Al Foil: 0.146 g Length of Al Foil: 5.99 cm Width of Al Foil: [missing value] Data Analysis Use your measured mass and volume](https://cdn.numerade.com/ask_images/78dc6c76e4624b5591bb6985b8044922.jpg)
SOLVED: The Thickness of Aluminum Foil Mass of Al Foil: 0.146 g Length of Al Foil: 5.99 cm Width of Al Foil: [missing value] Data Analysis Use your measured mass and volume

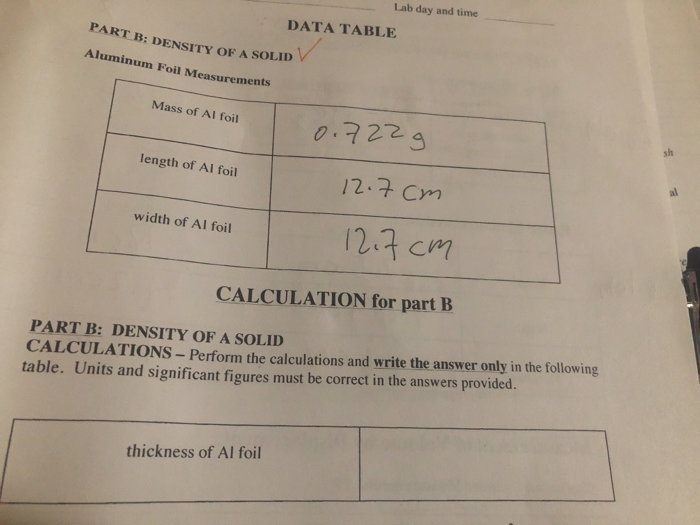
SOLVED: D) Thickness of Aluminum Foil Use the photos available on Canvas to complete this section: Mass of foil: Length of foil: Width of foil: L = 839 cm 15.55 cm 12
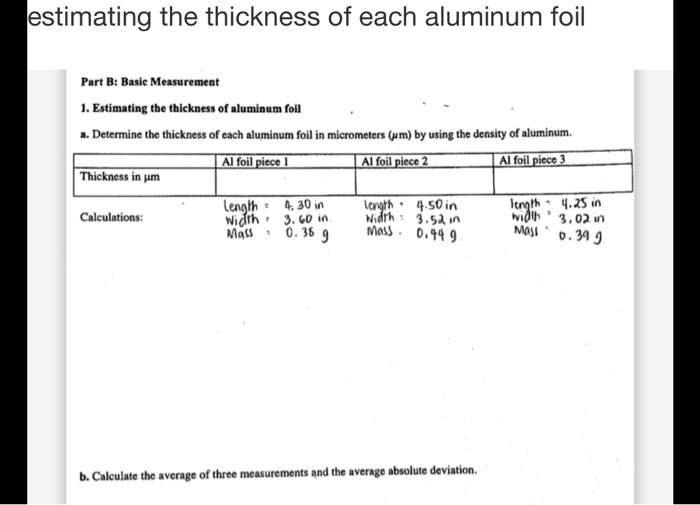
SOLVED: lestimating the thickness of each aluminum foil Part B: Basic Measurement Estimating tbe tbickness of alumioum foll Determine thc thickness of cach aluminum foil in micrometers (um) by using the dcnsity